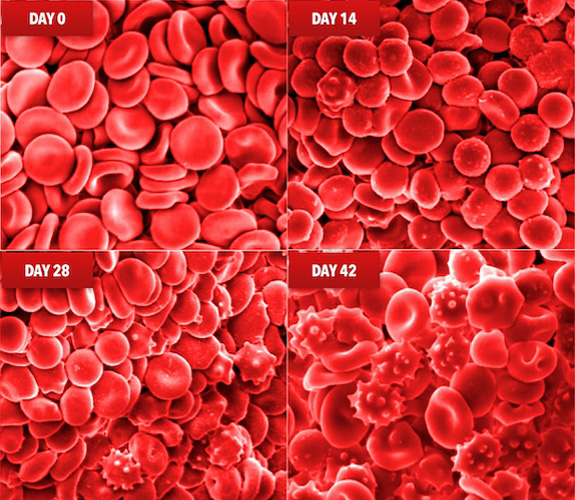
What is the Storage Lesion?
The storage lesion refers to the collection of changes that occur to red blood cells (RBCs) during storage.1,2,3
How Does the Storage Lesion Impact Red Blood Cells?
During storage, RBCs undergo progressive damage that may lead to:
- Reduced viability:
The ability of RBCs to survive and function effectively may decrease.3,5,6 - Impaired blood flow:
Changes in RBCs can affect their ability to flow smoothly through blood vessels.3,6,7 - Decreased oxygen delivery:
RBCs may become less efficient in delivering oxygen to the tissues.7,8,9 - Increased waste production:
Storage lesion can lead to the generation of potentially harmful waste particles.1,6,10
How Does the Storage Lesion Impact the Body During Transfusion?
- Changes from storage can mean fewer deformable red blood cells in circulation and more red blood cells that get stuck, resulting in less oxygen delivery to tissues. 7,11,12
- Free iron and hemoglobin can also damage blood vessels after transfusion.6,13
- These storage changes may play a role in poor outcomes in many types of patients requiring transfusions14,-21
See how RBCs affected by the storage lesion impact the body
Scientific Understanding
The existence of the storage lesion is well-established, and ongoing scientific research continues to deepen our understanding of its effects. These impacts underscore the importance of managing the storage lesion for effective transfusion outcomes.3,6,22-26
[1] D’alessandro A, Yoshida T, Nestheide S, et al. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion. 2020;9999;1–13. [2] Kim-Shapiro D, Lee J, and Gladwin M. Storage lesion. Role of red cell breakdown. Transfusion. 2011;51(4):844-851 [3] Yoshida T and Shevkoplyas S. Anaerobic storage of red blood cells. Blood Transfus. 2010; 8:220-36. [4] Mustafa I, Al Marwani A, Nasr K, Kano N, and Hadwan T. Time dependent assessment of morphological changes: leukodepleted packed red blood cell stored in SAGM. BioMed Research International. 2016; Doi: doi.org/10.1155/2016/4529434. [5] Yoshida T, Blair A, D’Alessandro A, et al. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017; 15 (2): 172-81. [6] Yoshida T, Prudent M, and D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019; 17 (1): 27-52. [7] Kor DJ, Van Buskirk C, and Gajic O. Red blood cell storage lesion. Bosnian Journal of Basic Medical Sciences. 2009;9(Supp 1):S21-S27. [8] Mohanty J, Nagababu E, and Rifkind J. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5(84). [9] Rabcuka J, Blonski S, Meli A, et al. Metabolic reprogramming under hypoxic storage preserves faster oxygen unloading from stored red blood cells. Blood Advance. 2022;6(18):5415-5428. [10] Torrance HD, Vivian ME, Brohi K, et al. Changes in gene expression following trauma are related to the age of transfused packed red blood cells. J Trauma Acute Care Surg. 2015;78:535–42. [11] Koshkaryev A, Zelig O, Manny N, Yedgar S, and Barshtein G. Rejuvenation treatment of stored red blood cells reverses storage-induced adhesion to vascular endothelial cells. Transfusion. 2009;49:2136-2143. [12] Anniss A and Sparrow R. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46(9):1561-1567. [13] Rapido F. The potential adverse effects of haemolysis. Blood Transfus. 2017;15(3):218-221. [14] Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229-39. doi: 10.1056/NEJMoa070403. [15] Sanders J, Patel S, Cooper J, et al. Red blood cell storage is associated with length of stay and renal complications after cardiac surgery. Transfusion. 2011;51:2286-2294. [16] Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570-572. [17] Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. Doi: 10.1186/cc8050. [18] National Heart Lung and Blood Institute. How is thalassemia treated? June 1, 2022. Accessed March 25, 2024. https://www.nhlbi.nih.gov/health/thalassemia/treatment. [19] Centers for Disease Control and Prevention. 5 steps to safer blood transfusions if you have sickle cell disease. July 6, 2023. Accessed March 25, 2024. https://www.cdc.gov/ncbddd/sicklecell/betterhealthtoolkit/blood-transfusions.html. [20] Germing U, Oliva EN, Hiwase D, and Almeida A. Treatment of anemia in transfusion-dependent and non-transfusion-dependent lower-risk MDS: current and emerging strategies. Hemasphere. 2019;30(6):e314. doi: 10.1097/HS9.0000000000000314. [21] American College of Surgeons. ACS TQIP massive transfusion in trauma guidelines. October 2014. Accessed March 25, 2024. https://www.facs.org/media/zcjdtrd1/transfusion_guildelines.pdf. [22] Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43(1):51-59. doi: 10.1016/j.transci.2010.05.009. [23] Burns JM, Yoshida T, Dumont LJ, et al. Deterioration of red blood cell mechanical properties is reduced in anaerobic storage. Blood Transfus. 2016;14(1):80-8. doi: 10.2450/2015.0241-15. [24] Zimring JC. Established and theoretical factors to consider in assessing the red cell storage lesion. Blood. 2015;125(14):2185-2190. doi: 10.1182/blood-2014-11-567750. [25] D’Alessandro A, Reisz JA, Zhang Y, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3(6):884-896. doi: 10.1182/bloodadvances.2018029629. [26] Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92(1):22-31. doi: 10.1111/j.1423-0410.2006.00860.x.